Bond formed by transferring electrons - usually forms between a metal and a non-metal. This activity supports students understanding of.

Electron Configurations The Cavalcade O Chemistry
Ge 1s 22s 2p63s 3p64s23d104p2 PART B SHORTHAND ELECTRON CONFIGURATION Use.

. They are in column 2 of the periodic table. Bond formed by sharing electrons - usually forms between 2 or more non-metals. Predict the relative reactivity of an atom based on its electron configuration and placement on the periodic table.
The neutral atom chlorine Z17 for instance has 17 electrons. Chlorine is found in the group 17 the halogens on the periodic table. An energy level with 8 electrons is stable.
1s2 2s2 2p6 3s2 3p6 4s2 3d6. Electron Configuration Li 1 3 1 1s2 2s1 N 5A 7 5 1s2 2s2 2p3 F 7A 9 7 1s2 2s2 2p5 Ne 8A 10 8 1s2 2s2 2p6 Na 1 11 1 1s2 2s2 2p6 3s1 Mg 2 12 2 1s2 2s2 2p6 3s2 Al 3A 13 3 1s2 2s2 2p6 3s2 3p1 Cl 7A 17 7 1s2 2s2 2p6 3s2 3p5 Ar 8A 18 8 1s2 2s2 2p6 3s2 3p6 K 1 19 1 1s2 2s2 2p6 3s2 3p6 4s1 Ca 2 20 2 1s2 2s2 2p6 3s2 3p6 4s2 Br 7A 35 7 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. P 1s2 22s2 32p6 3s 3p 3.
The main properties that can be compared is the melting. 72 The Modern Periodic Table. They are in column 1 of the periodic table.
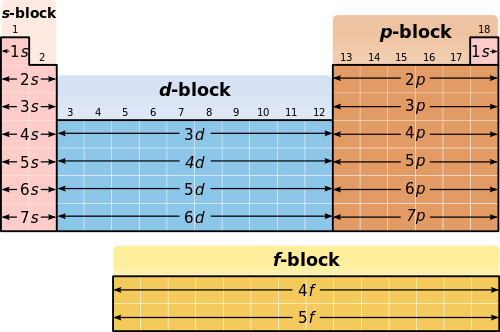
One of the many patterns contained in the periodic table is that of electron configuration. Write the electron configuration for the first 20 elements of the periodic table. Symbol e- Orbital Diagram and Longhand Electron Configuration 1.
1s2 2s2 2p6 3s1. Up to 24 cash back The atomic number of the elements on the periodic table are organized chronologically starting with Hydrogen with the the atomic number of 1 going from left to right. The atomic number is the number of protons in the nucleus of an atom therefore it is the same to the charge number of the element.
Write the electron configuration for the element bismuth atomic number 83. The electronic configuration in shell notation is given for an atom of each of the elements. In this activity you will identify these patterns.
Correlate the valence orbital of an atom with the atoms placement on the periodic table. As you complete the activity keep the following in mind. The electrons in the valence shell highest energy level are given in red.
Boron B has an electron configuration 1s²2s²2p¹. Dividing line on periodic table between metals and non-metals. Elements are combined in a definite ratio to form a compound.
Therefore its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. The anomalous electronic configuration of chromium and copper is interpreted as the displacement of 1 electron from an s orbital into a d orbital. The electron configuration follows a periodic order where lower-level shells are filled in before higher-level shells.
V 1s2 6 2s2 2p 3s2 3p6 4s23d3 4. How many electron configurations does a chlorine atom have. Ns2np6configurationexception-He Transition elements - 1B 3B - 8B d- block Lanthanidesactinides - f-block.
Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Similarly the configuration of Cu is Ar 4s 1 3d 10 instead of Ar 4s 2 3d 9. Pure substance that can not be physical or chemically deprecated into a simpler substance.
Uses the layout of the periodic table to do electronic configurations for atoms. The table below for the main group elements is set out just like the Periodic Table of the elements. These elements all have half filled or fully filled s-subshells that is s1 or s2 as the hightest energy subshell configuration.
The chloride ion Cl on the other hand has an additional electron. Classification of Elements Main group elements - representative elements Group 1A- 7A Noble gases - Group 8A all have. The Modern Periodic Table.
The periodic table as an organisational tool to identify patterns and trends in and relationships between the structures including electronic configurations and atomic radii and properties including electronegativity first ionisation energy metallicnon-metallic. The names of groups and periods on the periodic chart are alkali metals alkaline earth metals transition metals halogens and noble gases. The periodic table is arranged in order of increasing atomic numbers.
SOLUTION We can do this by simply moving across the periodic table one row at a time and writing the occupancies of the orbital corresponding to each row refer to Figure 629. For example the electron configuration of Cr is Ar 4s 1 3d 5 rather than Ar3s 2 3d 4 as we might have expected. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6.
GMT66 Building The Periodic Table349428349428 AnonymousAnonymous User2falsefalse article topic hypothesis yes showtoc yes license ccbyncsa authorname anonymous source chem 17398 source chem 17398 article topic hypothesis yes showtoc. First row 1s 2 Second row 2s 2 2p 6 Third row 3s 2 3p 6 Fourth row 4s 2 3d 10 4p 6 Fifth row 5s 2 4d 10 5p 6. Excepting helium elements with 2 valence level electrons Be and Mg have an ionic charge of 2 and lose 2 electrons.
Elements with 1 valence level electron H Li Na have an ionic charge of 1 and lose 1 electron. Copyright McGraw-Hill 2009 8. Electron configurations are the summary of where the electrons are around a nucleus.
Trends in the Number of Valence Electrons. One way to check if the notation is correct for a given element is to see if the sum of the exponents in the notation equals the number of electrons in an atom of that element. Metalloids have properties of metals and nonmetals.
These two elements have only one electron in the 4 s subshell because the second electron was promoted into a 3 d subshell. Note that these electron configurations are given for neutral atoms in the gas phase which are not the same as the electron configurations for. Og 118 oganesson.
Write the electron configuration of an atom using the Aufbau Principle. Mg 1s 22s2 2p6 3s 2. As we learned earlier each neutral atom has a number of.
Period row Group column Use the table on your book cover which shows only. This example warns you that there are exceptions to the general pattern of electronic. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Later you will use these patterns to determine the order in which electrons fill the orbitals of an atom. Noble gases have 8 electrons in their valance so they are stable and do not react with other elements. A close inspection of the periodic table shows that the electron configurations of certain elements appear to violate the rules.
The anomalous behavior is largely a consequence of the.

Electron Configurations In Atomic Energy Levels Video Lesson Transcript Study Com
Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry
How To Use The Periodic Table To Write Electron Configurations For Each Element Quora

Electron Configurations And Magnetic Properties Of Ions Introduction To Chemistry

Electron Configuration And The Modern Periodic Table Examples Pedia

Writing Electron Configurations Using Only The Periodic Table Youtube

Dublin Schools Lesson Electron Configurations Using The Periodic Table

0 comments
Post a Comment